IVC Filter Lawsuit Claims Against Cook Medical Continue With Trial Scheduling
October 27, 2015 (PRLEAP.COM) Health News
IVC Pulmonary Embolism, Inferior Vena Cava Perforations And Other Life-Threatening Injuries Alleged In IVC Filter Lawsuit Claims Reports Southern Med LawOctober 27, 2015 - IVC filter lawsuit claims filed against Cook Medical move forward with trial dates scheduled for for later in next year. There are currently over 130 Inferior vena cava filter (IVC) lawsuits pending litigation involving Cook Celect and Cook Gunther Tulip. The lawsuits allege the inferior vena cava filters can tilt, break or fracture, and punctures a patient's heart or lungs. The complaints further claim that Cook Medical failed to warn doctors and patients of the IVC filter risks.
The firm notes that attorneys for plaintiffs and defendants were to select five cases each for the discovery pool by October 5, 2015, according to a Case Management Order issued by Judge Richard L. Young, who is presiding over the multidistrict litigation in the U.S. District Court, Southern District of Indiana. After the 10 IVC filter lawsuit claims complete the discovery process, both sides are to select two potential cases for trial and inform the court in March 2016 of their selection. (In Re: Cook Medical, Inc., IVC Filters Marketing, Sales Practices And Product Liability Litigation – MDL No. 2570)
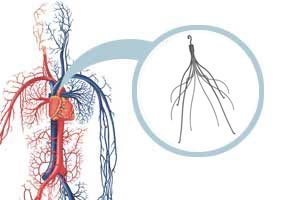
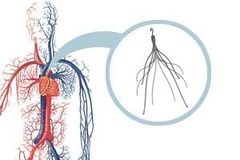
Retrievable IVC filters are small devices implanted into the inferior vena cava (the main vessel returning blood from the lower half of the body to the heart) to capture blood clots and prevent them from causing a pulmonary embolism (a blood clot in the lungs). The filters are designed to be removed once the threat of blood clots has subsided. The filters are used by patients at risk of a pulmonary embolism and those who cannot take blood thinners. [fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm221676., FDA, August 9, 2010]
In August 2010, the U.S. Food and Drug Administration (FDA) issued a warning about IVC filters after announcing that the agency had received 921 adverse event reports since 2005. According to the FDA, patients complained of the IVC filters migrating from their original position, components detaching from the device, filters fracturing and piercing the inferior vena, and the filters being difficult to remove. The FDA recommended that doctors remove the filters as soon as the risk of pulmonary embolism has subsided.
[fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm221676., FDA, August 9, 2010]
Court documents indicate that all federally filed IVC Filter Lawsuit Claims involving Cook Medical's IVC filters were consolidated for pretrial proceedings in the Southern Indiana district court in October 2014. A case was recently filed on behalf of a Michigan man who had a Cook Medical Celect filter implanted in 2010 to prevent blood clots from reaching his lungs after knee surgery. The plaintiff began experiencing sharp pains in his chest in November 2014. In that same month, the pain was so intense that the plaintiff lost consciousness while driving and ran off the road. After undergoing four emergency surgeries, doctors discovered a thin wire from the Cook IVC filter was piercing his heart and each heart beat lacerated his heart even more, according to the complaint. (In Re: Cook Medical, Inc., IVC Filters Marketing, Sales Practices And Product Liability Litigation – MDL No. 2570)
Southern Med Law is currently evaluating legal claims on behalf of patients from around the country who experienced side-effects from IVC filters, including perforation of the heart, lung or other organs, punctured vena cava, perforated aorta and death. If you believe you have been injured by IVC filters contact one of the firm attorneys for a free legal evaluation by calling 205-515-6166 or visit southernmedlaw.com for more information on this and other defective medical devices and to fill out a contact form.
About Southern Med Law And Filing a Cook IVC Filter Lawsuit: Throughout his career, Dr. Blaudeau has worked hard to develop a strong reputation in healthcare litigation. His first-hand knowledge of medicine has made the Southern Med Law team an aggressive and effective advocate for those who were harmed due to negligent medical device manufacturers. If you or a loved one were injured by a Bard retrievable IVC filter, please contact Southern Med Law today to learn more about your legal rights. Call today for a free, no obligation Bard IVC filter lawsuit review by filling out our online form, or by calling the office directly at 205-547-5525.
Southern Med Law
François M. Blaudeau, MD JD FACHE FCLM Esquire
2224 1st Avenue North
Birmingham, Alabama 35203
Phone: 205-547-5525
Cell: 205-515-6166
Fax: 205-547-5526
francois@southernmedlaw.com
http://www.southernmedlaw.com
Medical Negligence/MedicalDevice/Pharma/Qui Tam
Like us on Facebook
Follow us on Twitter
Join us on Google+
Pin with us on Pinterest