Morcellator Cancer Lawsuit News: Government To Investigate Power Morcellation Cancer Risk
September 08, 2015 (PRLEAP.COM) Health News
Southern Med Law is Currently Representing Women In Morcellator Cancer Lawsuit Claims The Purport Women Developed Uterine Cancer After Cell Spread When The Laparoscopic Power Morcellation Method Was Used During A Hysterectomy Or Myomectomy.September 8, 2015 - As morcellator cancer lawsuits continue to be filed around the country, The U.S. Government Accountability Office (GAO) has agreed to investigate claims that laparoscopic power morcellators spread cancer during gynecological surgeries after a request from Congress. Southern Med Law continues to represent women across the country who are believed to have had undetected uterine cancer spread after gynecological procedures involving power morcellators and had filed and settled the first power morcellator cancer lawsuit in a U.S. federal court for an undisclosed amount. (In Re: Power Morcellator Litigation, MDL Case No. 78)
GAO's managing director, Katherine Siggerud, sent a response on September 1 to U.S. Rep. Mike Fitzpatrick (R-Pa.), who led the12-member bipartisan Congressional group in asking for the probe. The GAO conducts investigations for the U.S. Congress. According to a report by Philly.com, Ms. Siggerud wrote, "GAO accepts your request as work that is within the scope of its authority." The legislators sent a letter to the GAO on August 7 noting that, "hundreds, if not thousands, of women in America are dead" because of power morcellators. The legislators asked the GAO to determine, among other things, whether the FDA's expedited approval process for medical devices "sufficiently identified" the cancer risks before the power morcellators entered the marketplace.
"Southern Med Law looks forward to the outcome of the GAO's investigation because the law firm is representing women who have been seriously or fatally injured allegedly by power morcellators," says Dr. François Blaudeau, founder of Southern Med Law. In July, Dr. Blaudeau settled the first power morcellator cancer lawsuit filed in federal court on behalf of a Pennsylvania widower, Scott Burkhart, whose wife, Donna Burkhart, 53, died of disseminated leiomyosarcoma in February 2013. Donna Burkhart developed the cancer after undergoing a power morcellator hysterectomy in March 2012. The lawsuit was filed in the U.S. District Court, Eastern District of Pennsylvania, against morcellator manufacturers, LiNA Medical APS, Kebomed AB & LiNA Medical US. The lawsuit was settled for an undisclosed amount.(Case No. 5:14-cv-1557)
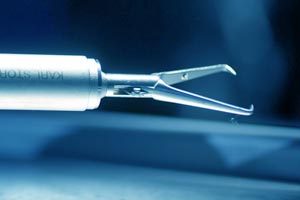
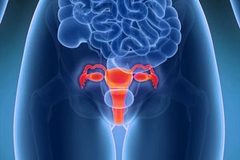
The law firm explains that laparoscopic power morcellators are used during a hysterectomy (removal of the uterus) and a myomectomy (removal of uterine fibroids). The device grinds uterine tissue into pieces so that the fragments can be removed through small (laparoscopic) incisions in the abdomen. It is during the removal of uterine tissue that undetected cancer cells are spread into the peritoneal cavity.
In November 2014, the FDA warned that power morcellators should be avoided in the majority of women who require a hysterectomy and a myomectomy due to the medical device's potential to spread undetected cancer cells which greatly reduced a woman's chances for long-term survival. The law firm notes that after the FDA's announcement, national health insurance companies including Aetna Inc. and UnitedHealth, announced that they would either end or restrict coverage for uterine morcellation procedures. Additionally, in the wake of the FDA's warning, the FBI decided to investigate power morcellators. According to CBS News, the FBI's probe includes questioning Johnson & Johnson, the largest power morcellator manufacturer, on what it knew about the cancer risks associated with the morcellators. Johnson & Johnson suspended global sales of the devices after the FDA's warning.
Court documents indicate that the U.S. Judicial Panel on Multidistrict Litigation (JPML) will hear oral arguments on October 1 on a request from six plaintiffs who filed a request to consolidate all federally filed power morcellator cancer lawsuits. The plaintiffs are asking the panel to transfer 22 pending morcellator lawsuits, and those that are subsequently filed, to the U.S. District Court, District of Kansas. The plaintiffs, who filed claims against various morcellator manufacturers, asked for a consolidation because all the complaints allege that power morcellators used during gynecological procedures can spread and upstage cancer. (In Re: Power Morcellator Litigation, MDL Case No. 78)
About Southern Med Law And Filing A Morcellator Cancer Lawsuit
Southern Med Law and Dr. François Blaudeau possess a unique understanding of the medical and legal questions at issue in power morcellator cancer lawsuit claims. It is this in-depth knowledge that is tantamount to a successful legal representation that protects the rights of the injured. The staff at Southern Med Law is not only trained in successfully handling your legal needs but also understand the pain and suffering and treatment from a medical point of view, and is committed to protecting the rights of all individuals. For more information on filing a morcellator cancer lawsuit contact Dr. Blaudeau by calling 1-205-515-6166 or visit www.southernmedlaw.com for more information and to fill out an online contact form.
Southern Med Law
François M. Blaudeau, MD JD FACHE FCLM Esquire
2224 1st Avenue North
Birmingham, Alabama 35203
Phone: (205) 547-5525
Cell: (205) 515-6166
Fax: (205) 547-5526
francois@southernmedlaw.com
www.southernmedlaw.com
Medical Negligence/MedicalDevice/Pharma/Qui Tam